The decay of u-235 worksheet answers – Delving into the intricacies of radioactive decay, this discourse on the decay of U-235 unveils the intricacies of this phenomenon, exploring its applications, safety considerations, and environmental impact.
Radioactive decay, a process that transforms unstable atomic nuclei into more stable forms, lies at the heart of understanding U-235 decay. This process involves the emission of particles or energy, resulting in the formation of new elements.
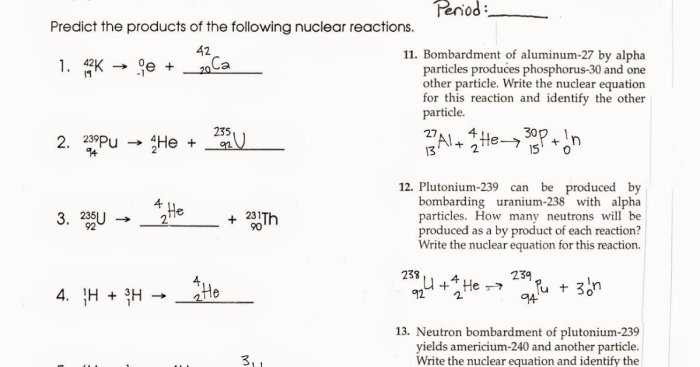
Decay of U-235: The Decay Of U-235 Worksheet Answers
Uranium-235 (U-235) is a radioactive isotope of uranium that undergoes a process called radioactive decay. Radioactive decay is the spontaneous transformation of an unstable atomic nucleus into a more stable nucleus, accompanied by the emission of radiation.
The process of U-235 decay involves the emission of an alpha particle (helium nucleus) and gamma rays. The alpha particle consists of two protons and two neutrons, and its emission reduces the atomic number of the nucleus by 2 and the mass number by 4. The gamma rays are high-energy photons that do not affect the atomic number or mass number of the nucleus.
Factors Affecting the Rate of U-235 Decay, The decay of u-235 worksheet answers
The rate of U-235 decay is constant and is not affected by any external factors such as temperature, pressure, or chemical environment. The rate of decay is determined solely by the inherent properties of the U-235 nucleus.
Half-Life of U-235
The half-life of a radioactive substance is the time it takes for half of the radioactive atoms in a sample to decay. It is a fundamental property of each radioactive isotope and is used to characterize the decay rate of the substance.
The half-life of U-235 can be calculated using the formula:
$$t_1/2 = \frac\ln 2\lambda$$
where:
- $t_1/2$ is the half-life
- $\lambda$ is the decay constant
The decay constant for U-235 is 9.8485 x 10^-10 per year.
Substituting this value into the formula, we get:
$$t_1/2 = \frac\ln 29.8485 \times 10^-10 \text yr^-1 = 7.038 \times 10^8 \text yr$$
Therefore, the half-life of U-235 is 7.038 x 10^8 years.
The half-life of a radioactive substance is significant because it determines the rate at which the substance decays. The shorter the half-life, the faster the substance decays. The longer the half-life, the slower the substance decays.
In the case of U-235, its long half-life means that it decays very slowly. This makes it a suitable material for use in nuclear reactors, where a slow and controlled decay rate is desired.
Applications of U-235 Decay
The decay of uranium-235 (U-235) has significant applications in various fields, particularly in the generation of energy and the development of nuclear weapons.
Nuclear Power Plants
In nuclear power plants, U-235 decay is harnessed to produce electricity through a process called nuclear fission. When a neutron is absorbed by a U-235 nucleus, it splits into two smaller nuclei, releasing a large amount of energy in the form of heat.
This heat is used to boil water, generating steam that drives turbines connected to electrical generators.
Nuclear Weapons
U-235 decay is also utilized in nuclear weapons. In an atomic bomb, a rapidly assembled critical mass of U-235 undergoes a chain reaction of fission, releasing an enormous amount of energy in a brief period. This explosive force is responsible for the destructive power of nuclear weapons.
Other Potential Applications
Beyond nuclear power and weapons, U-235 decay has potential applications in other fields:
- Medical Isotopes:U-235 decay can produce isotopes used in medical imaging and cancer treatment.
- Radioisotope Thermoelectric Generators (RTGs):U-235 decay generates heat that can be converted into electricity using RTGs, providing power for spacecraft and remote devices.
- Neutron Activation Analysis:U-235 decay produces neutrons that can be used to analyze the composition of materials.
Safety Considerations
The decay of U-235 is accompanied by the release of ionizing radiation, primarily in the form of alpha and gamma particles. These particles can be harmful to human health if not properly managed.
To mitigate these hazards, a comprehensive safety protocol is implemented. This protocol includes:
Handling and Storage Guidelines
- U-235 materials must be handled with appropriate shielding and protective equipment.
- Storage facilities must be designed to minimize radiation exposure and prevent unauthorized access.
- Regular monitoring of radiation levels is essential to ensure compliance with safety standards.
Environmental Impact
The decay of U-235 can have both positive and negative environmental impacts. On the one hand, it can release harmful radiation and radioactive isotopes into the environment. On the other hand, it can also be used to generate electricity and power homes and businesses.
Potential Risks
- Radiation exposure:The decay of U-235 can release harmful radiation into the environment. This radiation can cause cancer and other health problems in humans and animals.
- Radioactive waste:The decay of U-235 produces radioactive waste, which must be disposed of safely. If radioactive waste is not disposed of properly, it can contaminate the environment and pose a health risk to humans and animals.
Potential Benefits
- Electricity generation:The decay of U-235 can be used to generate electricity. This electricity can power homes and businesses, and it can help to reduce our dependence on fossil fuels.
- Medical applications:The decay of U-235 can be used to produce medical isotopes. These isotopes are used to diagnose and treat a variety of diseases, including cancer.
Strategies for Minimizing Environmental Impact
- Proper disposal of radioactive waste:Radioactive waste must be disposed of safely in order to minimize the risk of environmental contamination.
- Use of radiation shielding:Radiation shielding can be used to protect people and the environment from exposure to harmful radiation.
- Development of new technologies:New technologies are being developed to minimize the environmental impact of U-235 decay. These technologies include new methods for disposing of radioactive waste and new ways to generate electricity from U-235.
Detailed FAQs
What is the significance of the half-life of U-235?
The half-life of U-235, approximately 704 million years, determines the rate at which it decays. This knowledge is crucial for predicting the behavior of U-235 in various applications, such as nuclear power plants.
How is U-235 decay utilized in nuclear power plants?
Nuclear power plants harness the energy released during U-235 decay through a controlled chain reaction. This process generates heat, which is then used to produce steam and drive turbines, ultimately generating electricity.
What safety measures are implemented to mitigate the hazards associated with U-235 decay?
To ensure safety, U-235 is handled and stored in specialized facilities designed to prevent criticality, minimize radiation exposure, and contain any potential releases. These facilities adhere to strict regulations and employ multiple layers of protection.