Arrange these compounds by their expected solubility in hexane c6h14. – Embarking on an exploration of the solubility of various compounds in hexane C6H14, this discourse delves into the intricate relationship between molecular structure and solubility, unveiling the factors that govern the extent to which substances dissolve within this nonpolar solvent.
As we delve deeper into this topic, we will uncover the significance of polarity, molecular weight, and intermolecular forces in determining the solubility of compounds in hexane. Through a comprehensive examination of real-world examples, we will gain a profound understanding of the interplay between molecular characteristics and solubility behavior.
Solubility in Hexane: Arrange These Compounds By Their Expected Solubility In Hexane C6h14.
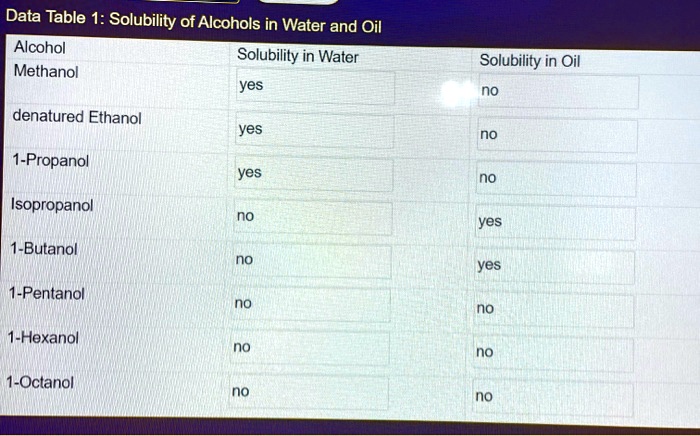
Solubility refers to the ability of a substance to dissolve in a solvent. Hexane (C 6H 14) is a nonpolar hydrocarbon solvent commonly used in organic chemistry. The solubility of a compound in hexane is influenced by its molecular structure and polarity.
Polar compounds, such as those containing charged groups or functional groups with a large dipole moment, tend to be less soluble in nonpolar solvents like hexane. Conversely, nonpolar compounds, such as hydrocarbons and alkanes, are more soluble in hexane due to their similar molecular structures and lack of polarity.
Factors Affecting Solubility, Arrange these compounds by their expected solubility in hexane c6h14.
- Molecular Structure:Compounds with similar molecular structures to hexane are more soluble in it.
- Polarity:Polar compounds are less soluble in nonpolar solvents like hexane.
- Molecular Weight:Larger molecules tend to be less soluble in hexane.
- Temperature:Solubility generally increases with increasing temperature.
- Pressure:For gases, solubility increases with increasing pressure.
Examples of Compounds and Their Solubility
| Compound | Molecular Structure | Polarity | Expected Solubility |
|---|---|---|---|
| Hexane | CH3(CH2)4CH3 | Nonpolar | High |
| Water | H2O | Polar | Low |
| Ethanol | CH3CH2OH | Polar | Moderate |
| Sodium chloride | NaCl | Ionic | Insoluble |
Methods for Determining Solubility
- Gravimetric Analysis:Measuring the mass of solute dissolved in a known volume of solvent.
- Titration:Adding a known amount of a reagent to a solution until a reaction occurs, indicating the endpoint.
- Spectrophotometry:Measuring the absorbance of light at a specific wavelength to determine the concentration of solute.
- Gas Chromatography:Separating and quantifying volatile compounds based on their solubility in a gas.
Applications of Solubility Data
- Drug Development:Determining the solubility of drugs to optimize their bioavailability and efficacy.
- Environmental Chemistry:Assessing the solubility of pollutants in water and soil.
- Industrial Processes:Designing and optimizing extraction and purification processes.
- Materials Science:Developing new materials with desired solubility properties.
Common Queries
What is the significance of polarity in determining solubility in hexane?
Polarity plays a crucial role in solubility, as hexane is a nonpolar solvent. Polar compounds, which possess a separation of charge, tend to be less soluble in hexane due to the weak interactions between their polar functional groups and the nonpolar hexane molecules.
How does molecular weight affect the solubility of compounds in hexane?
Molecular weight can influence solubility, with larger molecules generally exhibiting lower solubility in hexane. This is because larger molecules have a greater surface area, which results in stronger intermolecular interactions and reduced interactions with the hexane solvent.
What is the role of intermolecular forces in determining solubility in hexane?
Intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and van der Waals forces, play a significant role in solubility. Compounds with stronger intermolecular forces tend to be less soluble in hexane, as these forces compete with the interactions between the compound and the hexane solvent.